Shenzhen Cell Valley approved entry-exit special goods pilot enterprises "white list"
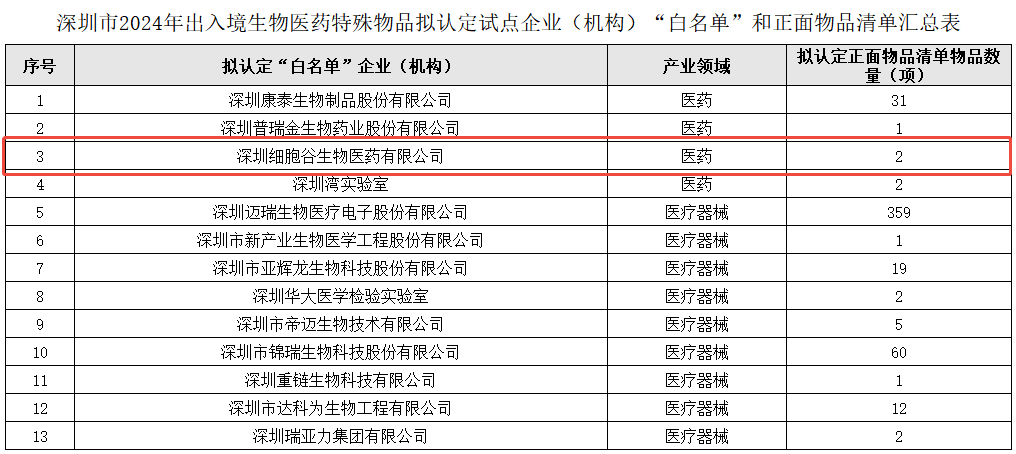
This selection is one of the important achievements of the pilot work plan of the joint supervision mechanism of entry-exit biomedical special goods in Shenzhen. The plan aims to optimize the entry and exit process of biomedical special goods by establishing a joint supervision mechanism, improve the efficiency of customs clearance, while ensuring the effectiveness of biosecurity and supervision. This initiative will not only help enhance the international competitiveness of Shenzhen's biomedical industry, but also provide more and better biomedical products and services for patients at home and abroad.
Looking forward to the future, Shenzhen Cell Valley will rely on the opportunity of being selected in the "white list", continue to increase scientific research investment, research the underlying core technology, and expand more international business at the beginning of 2025 to promote the continuous innovation and breakthrough of new cell therapy technologies.



